 Versono Medical Ltd.
Versono Medical Ltd.
 Versono Medical Ltd.
Versono Medical Ltd.
Versono Medical is a medical device startup company based in Galway.
They have developed an electro-mechanical device for endovascular surgery, known as FastWire.
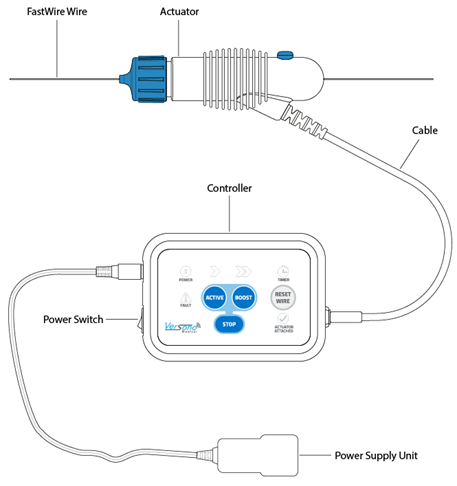
When I joined the company in 2021, there was a very basic proof-of-concept prototype, but no more.
There was also zero in-house expertise in the area of electronic engineering, or software development.
My task was to take the product concept and commercialise it.
At the time of writing, the FastWire device has successfully completed First-In-Human clinical trials and is going through the FDA's regulatory approval process.
| Responsibility | Achievements |
|---|---|
| Hardware Development |
|
| Software Development |
|
| Test & Verification |
|
| Other |
|
| Course | Presenter | Date | Days |
|---|---|---|---|
| Manual Handling | Clarke Consulting | TBC | TBC |
| Technical Writing | SQT | TBC | TBC |
| Medical Software Quality Assurance | University of Limerick / Irish Medtech Association | TBC | TBC |